Update 10 January 2012: Some of my descriptions of 3D television technology were, um, wrong
-ish. I speculated a bit to fill in some gaps and in one or two points I was mistaken. (see my correction
here.)
I'm afraid this will be another of my rambling posts, so let me apologize in advance if the narrative thread gets a bit lost in the exposition. This is intended to be -- and will eventually be -- a complaint about the intrusiveness of government. But before we can get to that there will be a lengthy Mr Wizard segment to establish some scientific background for my complaint. It is possible that you already know that stuff and can skip to the end. Here's a test question so you can decide: If a prism shows all the wavelengths of visible light -- red at one end, blue at the other -- then why does the color wheel wrap around in a circle? If you know the answer then hit the scroll wheel. If you are in the dark then...
Let There Be LightWhen you heat matter it becomes agitated. If it is solid its atoms vibrate. In a liquid they squirm like drunks doing the Conga. In a gas they fly around bumping into things like minor characters trying to drive home after a conga party in a Jetsons cartoon.
Since the atoms contain electrically charged bits -- electrons and protons -- all of this random acceleration, deceleration and jiggling about makes them give off electromagnetic radiation. Not all of the atoms are equally excited. As the party heats up, more and more are out on the dance floor but there are always some chilling out by the punch bowl.
A statistical measure of the average level of excitement is called the "temperature" but among individual atoms there is a random distribution of levels of excitation. Some are more excited than the average, some less. The really excited, seven-Red-Bull atoms give off high-energy radiation which has shorter wavelength and is generally bluer, while the laid-back atoms give off lower-energy, longer-wavelength, redder radiation.
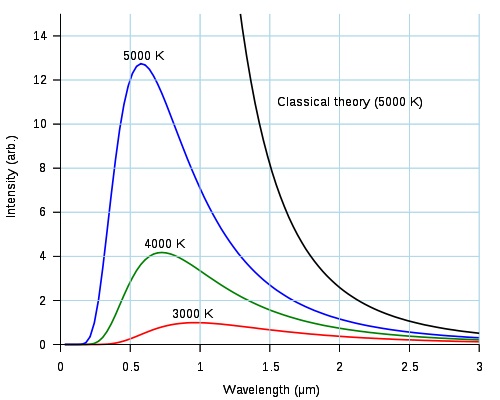
Light from thermal radiation is always a mixture of all the colors -- hotter objects have more shorter-wavelength (bluer) light in the mix but all the colors are there, just in different proportions depending on the temperature.
Here is a diagram from Wikipedia. It has three lines (the red, green and blue ones) that show the emission spectra of a hot body at three temperatures. It also has a black line labeled "Classical theory: (5000 K)" that I don't understand and request that you ignore.
We tend to think of sunlight as 'white', so a pretty good standard for white light would be light with the same mix of intensities at all wavelengths as sunlight. For sunlight at noon that would be approximately 5000K (the blue line above).
At low angles, just after sunrise or just before sunset, sunlight has to pass through more of the atmosphere than at noon. The blue light tends to scatter along the way leaving the light that reaches the ground redder and redder as the sun nears the horizon. When the sun sets on Manhattan the light appears red because the blue light has been scattered to make the sky blue over New Jersey.
Just before sunset the apparent color temperature of the sun is closer to 3000K which is also the approximate color temperature of ordinary incandescent light bulbs. Our brains adjust our color perception for the spectral changes in the light as the sun rises or sets. It can adjust to tungsten light bulbs and their light looks 'white' to us too.
Interestingly, while a mix of all the colors of visible light is a way to get light that looks white to us, it's not the only way. I'll get back to that later. It turns out we can't really see
all the colors of light. Basically, we only see three. Humans with normal vision see the world as a mixture of three colors. People with color blindness may see two colors, or even just one. There is a very small percentage of the population that sees four colors but they are oddities and don't really change the argument.
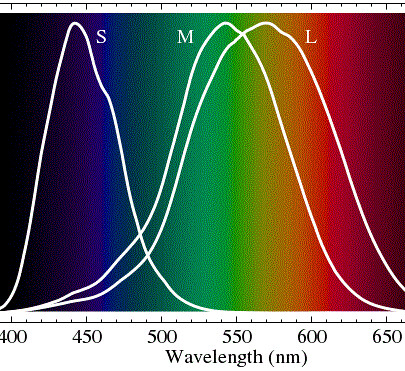
Our retinas have three kinds of 'cone' cells, each of which is sensitive to a range of colors, approximately 'red', 'green' and 'blue'. They are represented in the diagram below in terms of the wavelength of light: (S)hort=blueish, (M)edium=greenish, (L)ong=reddish. It is probably squeamishness about the "-ish" parts that kept the Wikipedia entry on 'Color Vision' from labeling them simply as blue, green and red.
These cone cells that give us color vision are themselves color blind. An "L" type cell that responds to reddish light reports a single value to the optic nerve: how much total reddish light is present. It doesn't report how red the light is, just how much light there is, in total, in the frequency band to which it responds.
You can map that value to a scale of, say, zero to ten, where zero is no red light detected and ten is the brightest red light you can distinguish. If you write down the numbers for the L (reddish), M (greenish), and S (blue) cone cells at some point on your retina then you have a set of three numbers that tell exactly what color you see there.
 Sunlight in the simplified model.
Sunlight in the simplified model.For the rest of this discussion I will use a slightly oversimplified model of color vision. If you look at the diagram above you will notice that the spectral sensitivities of the L and M cone cells overlap over a broad range of wavelengths. Unless you are red-green colorblind your brain sorts out the overlap for you and you see red light as a very distinct color from green light despite the fact that both kinds of cone cells respond to red, orange, yellow and green light perfectly well, only in slightly different degrees. It is only in the very red red light at one end of the spectral band, or in the teal colored light at the other, where the difference in sensitivities between the L and M cone cells is large. Having noted the overlap I will, for the balance of this discussion, ignore it and speak of the three colors of light we see as Red, Green and Blue (in that order longer wavelengths first).
Color TV, 2D and 3D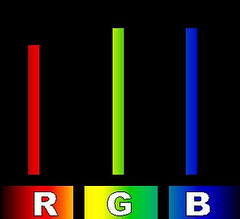 Sunlight on TV
Sunlight on TVIt is no coincidence that the three colors -- red, green and blue -- are the colors of the little dots on the screen of a color television. If your TV wants to show you something white it turns on all three colors -- red, green and blue -- each of which stimulates one of the three kinds of cone cells in your eye. Since TVs aren't really that bright compared to sunlight let's call the red intensity six, and the green and blue about seven on our one to ten scale. That gives us an R-G-B color number of 6-7-7 which looks the same to us as a piece of white paper viewed in sunlight at noon -- not quite as bright, but the same apparent color.
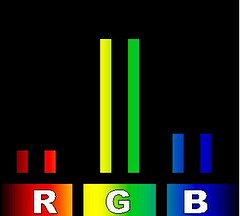 Green grass on a 3D TV
Green grass on a 3D TVBut is it the same color? Actually, no. Each of those three types of colored spots on your TV gives off light at a single wavelength. The red dots are a very particular red, the green a particular wavelength of green light and the blue a very precise blue. Manufacturers of some 3D TVs use this single-wavelength property in their displays. Instead of a single red color they use two at slightly different wavelengths. Similarly there are two greens and two blues. The dorky-looking glasses you wear to watch the TV have different lenses for the left and right eye. The lens for your right eye blocks one of the red wavelengths of light, one of the green wavelengths and one of the blue. The lens for your left eye blocks the other wavelength in each R-G-B pair.
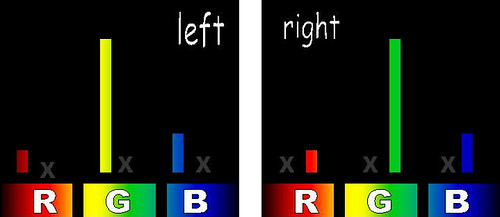 Left and right eye see the same color despite spectral differences.
Left and right eye see the same color despite spectral differences.The process is a bit more complicated than I am letting on (remember the Red/Green overlap) but the basic idea is that the TV can show your eyes two distinct spectral mixtures of light and, because they cause the same stimulation of the Red-, Green- and Blue-sensitive cone cells in in your eyes, you can't tell them apart. A spectroscope can tell you that they aren't the same color but your eye can't.
The Color Wheel Explained as a TriangleNow that we know that we only see three colors I am prepared to answer a question that has puzzled me for half a century, one which I have only just figured out while I was working on this post: If the colors of visible light span a linear spectrum from shorter to longer wavelengths, why does a color wheel wrap around in a circle?
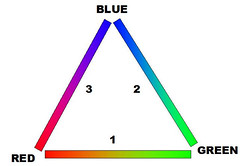 Color Triangle
Color TriangleLook at the triangle I have drawn on the right. If you were to inflate it so the lines bulge outward you would have the usual color circle. Looking at lines 1 and 2 you will see all the colors of the rainbow in the usual spectral ordering -- Red, Yellow, Green, Teal, Blue. Line 1 represents all possible combinations of Red and Green light with no Blue. Line 2 has combinations of Green and Blue with no Red. Line 3 has combinations of Red and Blue with no Green. With the exception of the endpoints -- pure Red and pure Blue -- line 3 contains all the colors that
aren't in the rainbow, most notably Purple. Rainbows don't have purple. Here's why:
A rainbow (or a prism) sorts out light according to wavelength. Each band is a particular single wavelength of light. There are bands for Red, Green and Blue, and between them fall bands of light with intermediate wavelengths. When our eyes receive light of a single wavelength that falls between the spectrally adjacent colors our cone cells can detect -- that is between red and green, or between green and blue -- then our eyes see that light as a mixture of the colors we can see -- orange/yellow/yellow-green for light between red and green and various shades of blue-green for light between green and blue. A 50/50 mixture of pure red light and pure green light looks yellow to us but so does light at a single wavelength half way between red and green. Both kinds of light produce the same stimulation in our cone cells. The same is true about wavelengths between green and blue, although there are some shades of blue-green light that we don't see particularly well.
But that is just for the spectrally-adjacent color ranges -- red/green and green/blue. The third side of our triangle -- red/blue -- mixes colors of light from both ends of the visible spectrum and our eyes don't see intermediate wavelengths of light as a mixture of those colors. A light at a single wavelength half way between red light and blue light is green light. So, when our eyes detect red light mixed with blue light, we see purple. There is no single wavelength of light that looks purple. Purple light is always a mixture of wavelengths. Since a rainbow sorts out light by wavelength and gives each spectral color its own band, there is no band for purple.
MetamerismColors that have the property that they are composed of different mixtures of wavelengths of light but cause the same stimulation of your cone cells and can't be distinguished are called metamers. When you look at a picture of an object -- a photograph, a video or even a painting -- and the colors look accurate to you, then those colors are almost certainly metamers of the colors of the objects being rendered. A real-life banana reflects yellow light as well as some red and some green (depending how ripe it is) but a banana on TV consists of a mix of red and green light because (most) TVs have no yellow dots.
 Fetching Green Handbag
Fetching Green HandbagYou don't have to be a couch potato to see metameric color matches. They happen all the time. The dyes and pigments we use to make objects the color we want them to be are chosen because they absorb light in particular wavelengths and reflect or transmit the rest (depending on whether the object is translucent or opaque.) On the right is a reflectance spectrum for a fetching green handbag tinted with pigments that absorb most wavelengths of red and blue light. It reflects a lot of green but there is other light there too. In sunlight its R-G-B number might be 2-5-2.
 Matching Green Suede Shoes
Matching Green Suede ShoesHere are the matching green suede shoes. They were dyed with chemicals that absorb light in the middle of the green band but reflect more light in the yellow and blue-green ranges. Despite the differences in spectral composition your eye still sees the same amount of generally red, green and blue light -- 2-5-2 -- and the shoes appear the same color...
in sunlight or incandescent light.The Incandescent Light Bulb BanBut as of January 1st, 2012 it will become illegal to manufacture and sell one-hundred watt incandescent light bulbs -- the type of incandescent used to light a room with a single bulb. You might have heard that Congress repealed the light-bulb ban but that isn't exactly true. Congress declined to provide any funds to enforce the new law -- in effect giving the 100 watt incandescent bulb a reprieve -- but the law remains on the books. Congress can easily restore funding at any time and the manufacturers know it.
Making incandescent light bulbs for the US market has become a risky economic decision. Companies will stop making them or scale back so they can't be caught with lots of unsellable inventory if funding for enforcement is restored. Prices of incandescent bulbs will go up. People will switch to compact fluorescent bulbs which are more efficient. They will spend less for electricity and can use that extra cash to buy antidepressants because the light in their living room suddenly reminds them of restrooms in bus stations. And that green handbag and those green suede shoes will never match again.
Why Compact Fluorescent Bulbs (still) SuckCompact fluorescent bulbs don't emit thermal radiation. They aren't hot. They generate light in a cascade of several steps. The first step is that a stream of electrons passes through the gas inside the glass tube -- a mixture of argon and mercury vapor -- which causes the atoms to change energy states. The electrons in the mercury atom have several discrete energy states they can assume but the higher energy states are unstable.
When a mercury atom gets zapped with a passing electron it jumps to one of those unstable, higher-energy states. It will then cascade through successively lower energy states until it reaches a stable state. Each time it jumps from a one state to another it sheds energy in the form of a photon. Depending on the state transition involved (what the before and after energy states are) that photon will have some very-exact amount of energy -- a very specific color. The most frequent state transitions with mercury emit light that is either blue or ultra-violet.
The second light-producing step in a compact fluorescent bulb happens when that ultra-violet light hits the rare-earth phosphors coated on the inside of the glass. The high-energy ultraviolet photon excites those atoms in pretty much the same way as the electron did with the mercury. The atoms in the phosphors have their own particular energy state transitions and each of those transitions emits a photon with a very specific color. The phosphors are selected to add some reds and greens to the mercury blues to give a light that looks white.
In any discussion of compact fluorescent lights it would be ungrateful and wrong to ignore the hard work of the lighting scientists who have been beavering away for decades to improve their light. They have several tricks they do with the phosphors that let them fill in between the colors a bit but I didn't describe those tricks because I don't understand them. But the spectral peaks associated with the energy level transition still predominate in the light. The light from older and/or cheaper CFLs is appallingly horrible but the newer, fancier CFL bulbs produce light that is merely rather bad.
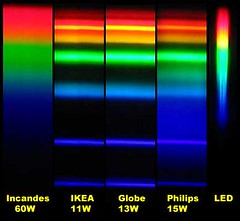
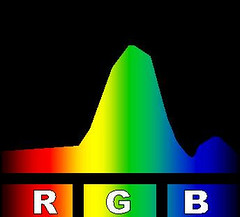
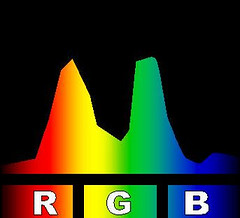
At right is an image that compares the spectra of several kinds of compact fluorescent light bulbs with a 60 watt incandescent bulb and with an unspecified light emitting diode-based bulb. The image is one I found somewhere on line. The page on which I found it neither claims nor references a copyright so I
borrowed the image to use here. The comparison makes the Philips brand bulb look pretty good so it might have come from them.
There are a few oddities about the comparison image that strike me as peculiar. It may have gotten a bit dented in its travels on the Internet. In particular the yellow bands seem to have wandered off from where you would expect them. The incandescent spectrum has almost no yellow band and the spectra for the fluorescents show a second yellow band right in the middle of the reds. But it gives you the idea; fluorescent lighting tends to have lots of light at a few very specific colors and not much in between.
Here's a better image to show that phenomenon:
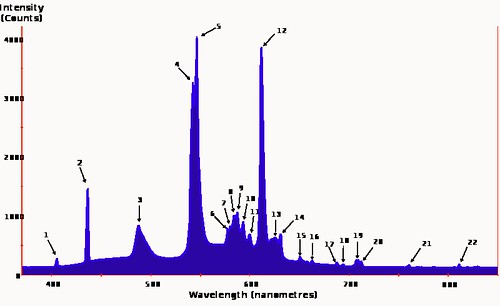 Fluorescent lighting spectrum peaks labelled from Wikipedia
Fluorescent lighting spectrum peaks labelled from WikipediaThe numbers that label the peaks in the graph refer to a list of elements that fluoresce at that particular wavelength, generally Mercury, Europium and Terbium. If you click on the link in the caption it will take you to a Wikipedia page that has the list. There are several other spectra for various sorts of fluorescent lights on the
Wikipedia page where I found the above. Among them is a spectrum for a Philips bulb which may be the one in the comparison image. It does show a somewhat better fill-in between the peaks but it doesn't label its Y axis and I am suspicious that they used a log transform to hammer down the peaks a bit. So I didn't use it here.
You may be wondering, if we only see three colors and (with the right rare-earth phosphors to balance the Mercury blue) the light from a fluorescent bulb stimulates all three types of cone cells in our eyes and looks white to us, then isn't the problem with fluorescent lights basically fixed? Actually, no. Remember the green shoes and handbag.
The shoes and handbag appear the same color in sunlight and under incandescent light because they are a metameric color match in that light. They reflect a different spectrum but we can't see the difference. Fluorescent lights have a big spike right in the middle of the greens. Our handbag reflects true greens strongly and is likely to show bright green under fluorescent light. The shoes, that make the same metameric green by straddling the wavelength, will appear duller, probably a bit brown.
You can simulate the experience of viewing things under compact fluorescent lighting as follows: First go to your neighborhood multiplex and watch the 3D movie of your choice. On your way out steal the glasses and wear them for the rest of the experiment. (You can return them later.) Next, find a room with an overhead fixture that takes three 60 watt light bulbs. Replace one of the bulbs with a black-light bulb that you buy at Spencer's gifts at the mall. Then take all the clothes from your closet, sit under the light wearing the glasses, and sort your clothes into piles that look good together. Finally, carry your piles out into the sunlight, take off your glasses and see what you think of your choices. I'll bet the result won't be pretty.
Light Emitting Diode LightingI won't say much about LED lighting except to say that LED bulbs seem to produce better light than CFLs but are very expensive, especially in the higher power ranges. Most LED light bulbs are 60 watt equivalents or lower although 100 watt replacement bulbs are entering the market now. They are very efficient -- better than CFLs and last pretty much forever. They cost 20 to 30 times as much as incandescents but because they are so efficient and last so long they can pay for themselves...
sometimes.
If you replace the incandescent bulb on your porch light that burns all the time with a LED bulb it might pay for itself in saved electricity in a year or so. If you replace the light bulb in your guest room closet, where the bulb burns 20 minutes a day, six days a year, that bulb will pay for itself when pigs fly.
Unleashing the Forces of the MarketOne theory about the light bulb ban states that with incandescent bulbs being illegal the increased demand for other, more efficient forms of lighting will cause manufacturers to focus on them for research and to compete with them on price. That is, the light bulb ban will make compact fluorescent lighting better and will make LED lighting cheaper. It could happen, I suppose. But at least one unsatisfactory precedent presents itself.
A Crappy PrecidentIn 1992 President George H. W. Bush signed the Energy Policy Act. If mandated toilets that consume no more than 1.6 gallons per flush. It went into effect in Jan 1, 1994 for residential buildings and Jan 1, 1997 for commercial buildings. It offers a nice parallel with the light bulb ban. At the time the law went into effect there were low-flow conventional toilets but they weren't very satisfactory. They are the analog of CFLs in our simile. The role of LED lighting was played by pressurized-tank and other pressure-assisted flush toilets which worked quite well with 1.6 gallons but were much too expensive for most people to buy.
So, in 1994 the Energy Policy Act unleashed the full innovative and competitive powers of the US market to solve the problem of producing a cost-effective, reliable toilet that used no more than 1.6 gallons of water per flush. The question is, how long did it take for the market to provide a common, economical residential toilet that a grown man can use confidently without knowing where the plunger was located?
Let's see, the "working toilet ban" took effect in 1994. This is 2012. That's something like 18 years so far. But I live in hope.